The Drosera peltata complex comprises a group of similar tuberous sundews with crescent-shaped leaves, an erect stem and a basal rosette. Together, these taxa form Drosera section Luniferae. The species in the complex have been subject to frequent revision, giving rise to conflicting taxonomic concepts amongst various authors and authorities. This article examines their convoluted taxonomic history and reconciles it with my own personal observations of the plants in the wild.
Of the currently described taxa, two species are distinguished by their glabrous sepals. in turn, the two are unambiguously separated from each other by seed morphology. Drosera lunata Candolle, A.P. de (Figure 1) describes plant with oval to round seeds broadly distributed across East, South and South East Asia, monsoonal Australia and down the east coast to Sydney. In Australia, the plants are generally golden to red in colouration and prefer sandy coastal habitats and drainage lines in savannah environments. The basal rosette is usually only produced on immature plants and has a thin, linear petiole with oval laminae. The flowers are usually white (rarely pink) and the stigmas are usually brown at their base.




Drosera auriculata (Backh. ex Planch) (Figure 2) also has glabrous sepals but differs in that it has very long seeds. It is found in cooler environments across SE Australia and New Zealand, where it grows in heathland, sclerophyll forest and seepages. The plants are usually green or olive in colour, although some mountainous forms are red. The flowers are most commonly pink (sometimes white) with styles that are uniformly pale in color. The basal rosette is often absent or greatly reduced in mature plants and vary in the thickness and length of the petiole considerably across its range.



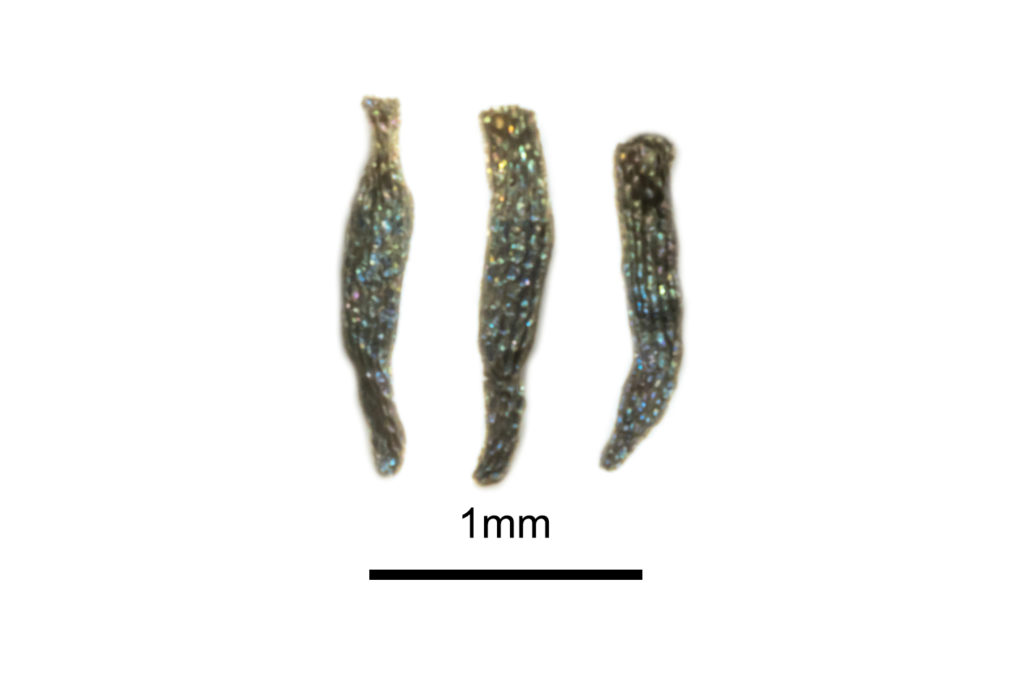
Two taxa are endemic to south-west Western Australia and are easily distinguished by their floral morphologies. Drosera yilgarnensis R.P.Gibson & B.J.Conn (Figure 3), was described in 2012 and was previously informally referred to as Drosera peltata ‘Western Australian form’. It is identifiable by its bright orange and highly divided sepal structure, glandular indumenta on its sepals and strong basal rosette. The plants are most commonly encountered in the Wheatbelt region, where it grows in the washes around granite outcrops.




Drosera bicolor Lowrie & Carlquist is variably placed within the complex. While it was originally described by Lowrie from plants informally referred to as Drosera peltata ‘Hammersley’, Gibson et al. (2012) consider it to be outside of the section based on its morphology. No published phylogenies have examined the genetic relationship of this species. The species is distinguished by its notched petals which each have a red dot at the base. A crowded basal rosette is produced but is often buried under sand. It grows in silica sand at the edge of saline watercourses between Jerramungup and Ravensthorpe.




The hairy-sepals plants of SE Australia are more taxonomically complicated due to differing species concepts presented by various authors, as well as inconsistencies amongst the Australian state herbaria. Across the region, I have observed two main groupings of hairy-sepaled plants – those that always produce strong basal rosettes and a shorter, more crowded inflorescence; and those with less robust to no basal rosette and a longer, less crowded inflorescence.
Of the plants with strong basal rosettes and short inflorescences, Drosera hookeri R.P.Gibson, B.J.Conn & Conran (Figure 5) is the least ambiguous. It was first described as Drosera foliosa by Hooker in 1948, but the name was invalid as there was no clear type specimen assigned, so Gibson et al. 2010 assigned a lectotype of a short, multibranched plant from the Midland region of Tasmania and renamed it Drosera hookeri. This species is recognised by its bright green foliage, very robust basal rosette in flowering specimens, branching nature, short stems, small white flowers and ‘bottle shaped’ seeds with a deeply pitted texture. Under my observation of the species in Victoria, it prefers winter-wet grassy clearings in disturbed woodland and the wet periphery of seasonal swamps.




Another similar taxon is present with a strong basal rosette in flowering specimens but has taller and less branched stems. In the 2012 publication, Gibson et al. included these plants within their concept of D. hookeri. Conversely, Lowrie in Magnum Opus (2012) included these plants under his concept of D. peltata. A resolution was presented by de Salas in 2018, who asserted that the tall taxon represented a separate species based on observed stable morphological characteristics. These plants, originally described by Planchon in 1848 as a subspecies of D. peltata, were elevated to species status as Drosera gunniana (Planch.) de Salas (Figure 6).
Having seen across VIC and NSW, I agree with de Salas’ view that Drosera gunniana should be separated from D. hookeri. Traits that can be used for its distinction from D. hookeri include a longer stem that branches few times if at all, larger flowers that are usually pink, stems and leaves that are usually olive in colour (rarely red) and smaller seeds that are less strongly ‘bottle’ shaped with a shallow texture. It tends to grow in compacted clay within open woodland and on mossy seepages atop rock outcrops. It should be noted that a similar taxon is present in coastal regions of central NSW to SE QLD but differs from the standard form in that it has sepal surfaces that are variably hairy, fimbriate sepal margins and vivid pink blooms. These plants are currently generally included under D. gunniana but may represent a new taxon.




The final group of plants are those with a long inflorescence with well-spaced, hairy flower buds and basal rosettes with thinner petioles that are sometimes absent.
The better defined of these two taxa is Drosera gracilis Hook.f. ex Planch (Figure 7.), described in 1848 from plants collected in Arthur’s Lake in the central Tasmanian highlands. These specimens are well-preserved, with an accompanying illustration that highlights obscure features, most notably the long seed that has a tapering appendage at the extremity. The illustrations also depict the red glands at the tip of the sepal hairs, which are only apparent under high magnification and with strong lighting. In general, the taxon is reddish in colour, slender, and usually produces a basal rosette with thin petioles in plants of flowering age. Lowrie in Magnum Opus (2013), also notes that the plants have a propensity to form lateral reproductive stolons.
I have observed plants that match the type specimen of D. gracilis across New South Wales and Victoria. In the southern to central mainland Great Dividing Range, it is common in subalpine creek systems. In NSW, it extends down to the coast where it prefers to grow in moss beds atop sandstone. In central Victoria, the species also grows in lowland heathland swamps, typically in areas that are waterlogged in winter. The aforementioned sepal hair glands and seed shape are consistent from the plants I’ve seen. I have directly observed reproductive stolons in plants from the Victorian High Country, but I do not regularly check for subterranean features. It also should be noted that the plants often grow in crowded colonies that are indicative of clonal reproduction.




The final described taxon in the complex is Drosera peltata Thunberg (Figure 8.). Despite being the first described species of the section, there are conflicting concepts for the species. For example, Gibson et al. (2012) synonymises D. gracilis with it, whereas Lowrie (2013) considers it the same as D. gunniana. Much of this debate stems from the poorly recorded and incomplete type material of Drosera peltata.
Drosera peltata was first described by Thurnberg in 1797 from material collected from Sydney. As was common at the time, the actual description was rather brief and insufficient to distinguish it from most other species within the complex. Adding to this, the holotype (a single physical specimen from which the species was described) was not designated or recorded. Conn in 1981 found a likely candidate in the Thunberg Herbarium and designated it as a lectotype, which now serves as the definitive example of D. peltata. This specimen represents a tall plant with hairy sepals and without a basal rosette. The trouble is that this lectotype had only just begun to flower and so lacks important characteristics such as inflorescence length and seed shape. Whether the sepal hairs are gland-tipped is unclear from the publicly available image.
Due to the morphologically incomplete lectotype, circumstantial evidence must be used in determining the identity of that specimen and by extension, the D. peltata species concept. Gibson et al. (2012) emphasize the origin of the lectotype, as it appears to have come from material collected in the Sydney region in the Smith herbarium. They note that more complete specimens in the Smith herbarium are similar enough such that they can be considered the same taxa. However, the herbarium sheets from the collection show at least three taxa from Sydney, so it is my opinion that more data is needed to confidently identify the lectotype.
An examination of wild plants within the Sydney region provides more evidence. In the Sydney basin today, there are at least five taxa within the complex present. In the first instance, Drosera auriculata and D. lunata can be excluded as they have glabrous sepals and the lectotype has hairy sepals. It is unlikely that the lectotype represents the east coast form of D. gunniana as a basal rosette is absent in the lectotype (D. gunniana was collected by Smith and his specimens seem to preserve the basal rosette features well). The lack of a basal rosette also somewhat excludes D. gracilis, which usually (although not always) has one. Through the process of elimination, I am circumstantially swayed to believe that the lectotype represents tall plants common in the sandstone-based heathlands of Sydney, hereafter referred to as D. peltata.
My personal concept of D. peltata is informed by my field examination of the taller heathland plants and similar specimens throughout the central Great Dividing Range. Drosera peltata is variable species. All plants have a well-spaced inflorescence, with sepals that are hairy to various densities. The sepal hairs usually lack a red gland at the tip at the time of flowering (although a small gland is sometimes present on immature buds and in some flowers). The seeds are oval-shaped and pointed at the ends. In sandy substrates within coastal heathland, the plants are usually olive-green to bronze in colour, about 20-30 cm in height, and often lack basal rosettes in flowering-sized specimens. In exposed niches such as shallow moss beds atop sandstone or cold, higher altitude sites in the central Great Dividing Range, D. peltata is generally shorter, redder and often has a well-developed basal rosette. It is typically associated with sandstone-based habitats.




Assuming that my concept of D. peltata matches Thunberg’s lectotype, the question then moves to whether D. peltata is different to D. gracilis. Most recently, Gibson et al. (2012), through the statistical clustering of 29 morphological traits, concluded that the two taxa were insufficiently distinct and so circumscribed D. gracilis under D. peltata. This is not surprising considering the wide morphological variation in D. peltata, in which the vegetative aspects can be more or less identical to D. gracilis in some locations. The only trait that is always different between the taxa is their seed shape. Secondary characteristics that usually, but do not always distinguish the two include the presence of obvious red sepal hair glands and stolon formation, however, these features were not included in Gibson’s analysis methodology.
One of the more salient limitations of purely morphologically based analyses is that habitat, geographical, seasonal and genetic information is ignored. When examining how both D. peltata and D. gracilis grow in the wild, further differences become apparent. Drosera gracilis prefers colder environments, including in subalpine creeks and seepages in the NSW and Victorian High Country, regularly extending into lowland swamps in the southernmost states. Conversely, D. peltata is mainly found along the warmer coast of central NSW to the Victorian border and adjacent mid-altitude ranges, where it primarily grows in sandstone-based heathland. Across its range, D. gracilis tends to emerge later in the season compared to sympatric taxa within the complex. In subalpine regions, its growing season is from spring to mid-summer, after the snow has melted. In comparison, D. peltata tends to grow throughout winter and spring. It should be noted that both taxa inhabit converging niches in the sandstone regions of the central Great Dividing Range, where the basal rosette, foliage colouration and growth period can be very similar between the two plants.
In considering their morphological and environmental characteristics, I consider that D. peltata and D. gracilis are as diverged from each other as the other species pairs within the complex. It is therefore my personal opinion that the two should be separated as distinct species. This is not to say that the other taxonomic views are inaccurate: the delineation between taxonomic ranks is inherently subjective as there is no universally accepted threshold for speciation agreed upon by authorities of the genus. In recent years, genomic sequencing has been increasingly used to delineate morphologically similar carnivorous plant taxa. It is my view that robust genomic sequencing and phylogenetic clustering, in conjunction with geographical and morphological information, is required to objectively and quantitatively resolve the relationships within the Drosera peltata complex and I look forward to emerging research in this area.
Important notes:
– The taxonomy of plants within the Drosera peltata complex is by no means complete and there remain many examples of undescribed taxa.
– The descriptions in this article are limited to the literature and my own observations of plants throughout Australia.
– The descriptions should be treated as generalisations. It is not uncommon to find populations with atypical traits.
– This article has not been peer-reviewed and represents my personal understanding of the complex.

























Table of characteristics for Drosera section Luniferae
| Species | Basal Rosette | Flowers | Stem | Sepals | Seed |
| Drosera auriculata | Generally present in immature plants. Petioles thin in low light, thicker in high light. Generally absent or reduced to bracts in flowering specimens. | Usually pink. Styles pink or white. | Usually unbranched in NSW. Branching common in Victoria. | Glabrous. | Very long and thin |
| Drosera bicolor | Small, tight rosette with very short petioles. | White with notched edges and red dot at center. Styles clustered into short clump. | Unbranched | Hairy. Hairs short and gland-tipped | Small and round |
| Drosera gracilis | Generally present in both immature and flowering specimens. Thin petioles. | White. Styles usually brown at the base. Flowers well spread and produced on a long inflorescence. | Unbranched | Hairy. Hairs tipped with a dewless red gland | Elongated with a tapering appendage at the extremity (Like a lady-finger banana) |
| Drosera gunniana | Always present. Robust basal rosette with thick petioles and large laminae. | Usually pink, sometimes light pink or white. Styles white. Flower buds comparatively large. Flowers clustered on a short inflorescence. | Sometimes sparingly branched near terminus, often unbranched | Hairy. Hairs long and thin. | Oval with a small lobed appendage at one extremity. Reticulated surface pattern with shallow texture. |
| Drosera hookeri | Always present. Robust basal rosette with thick petioles and large laminae. | Usually white. Styles white. Flower buds comparatively small. Flowers clustered on a short inflorescence. | Frequently branched on the lower two thirds of the main stem. | Hairy. Hairs shorter | Oval with a large lobed appendage at one extremity. Shaped like a stubby beer bottle. Reticulated surface pattern with deep texture. |
| Drosera lunata | Present in immature plants. Long, thin, straight petioles. Absent in flowering specimens. | Usually white, rarely light pink. Styles usually brown at base. | Sometimes sparingly branched near terminus. Often unbranched | Glabrous | Oval to round |
| Drosera peltata | Variable. Generally reduced or absent in large plants within heathland. Variably present or reduced in young plants or plants growing in cold/exposed environments and mossy seepages. Petioles thin. | White. Styles usually brown at base. Flowers well spaced and produced on a long inflorescence. | Unbranched | Hairy. Hairs usually do not have a gland. | Oval with a pointed end. |
| Drosera yilgarnensis | Always present. Robust basal rosette with large laminae. | White. Styles heavily divided and bright orange. | Sometimes sparingly branched near terminus. Often unbranched | Hairy. Hairs glandular | Round |
References and links for the original descriptions of Drosera section Luniferae
| Species | Reference | Link to descriptions |
| Drosera auriculata | Backh. ex Planch. Ann. Sci. Nat., Bot., sér. 3, 9: 295 (1848) | https://www.biodiversitylibrary.org/page/41549138 |
| Drosera bicolor | Lowrie & Carlquist. Phytologia 73:109-111,Fig.6 (1992) | biodiversitylibrary.org/page/12985415 |
| Drosera gracilis | Hook.f. ex Planch. Annals de Sciences Naturelles, Botanique 9: 297 – 298 (1848) | https://www.biodiversitylibrary.org/page/41549140 |
| Drosera gunniana | (Planch.) de Salas. Muelleria 36: 92 (2018) -Planchon, J.E. 1848: Sur la famille des Droséracées. Annales des Sciences Naturelles; Botanique Sér. 3: 285–309. | – D. gunniana https://www.biodiversitylibrary.org/partpdf/291978 – D. peltata subsp. gunniana https://www.biodiversitylibrary.org/page/41549140 |
| Drosera hookeri | R.P.Gibson, B.J.Conn & Conran. J. Adelaide Bot. Gard. 24: 39 (2010) – Drosera foliosa Hook.f. ex Planch. Ann. Sci. Nat., Bot. sér. 3, 9: 298 (1848), nom. illeg. | – D. hookeri. https://www.jstor.org/stable/23874283 – D. foliosa. https://www.biodiversitylibrary.org/page/41549141 |
| Drosera peltata | Thunberg, C.P. Dissertatio Botanica de Drosera: 7 (1797) B.J.Conn J. Adelaide Bot. Gard. 3:91-100 (1981) | https://bibdigital.rjb.csic.es/records/item/14488-dissertatio-botanica-de-drosera https://www.jstor.org/stable/23872356 |
| Drosera lunata | Candolle, A.P. de. Droseraceae. Prodromus Systematis Naturalis Regni Vegetabilis 1: 319 (1824) | https://www.biodiversitylibrary.org/page/154275 |
| Drosera yilgarnensis | R.P.Gibson & B.J.Conn. Austral.Syst.Bot. 25:75-76, Fig. 14 (2012) | www.researchgate.net/publication/230752629 |
References to Literature:
Gibson, R. P., Conn, B. J., & Conran, J. G. (2010). Drosera hookeri R.P.Gibson, B.J.Conn & Conran, a replacement name for Drosera foliosa Hook.f. ex Planch., nom. illeg. (Droseraceae). Journal of the Adelaide Botanic Garden, 24, 39–42. http://www.jstor.org/stable/23874283
Gibson, R., Conn, B. J., & Bruhl, J. J. (2012). Morphological evaluation of the Drosera peltata complex (Droseraceae). Aust. Syst. Bot., 25(1), 49–80. doi: 10.1071/SB11030
De Salas, M. F. (2018). Drosera gunniana comb. et stat. nov., a species in the Drosera peltata (Droseraceae) complex. Muelleria, 36. doi: 10.5962/p.291978
Conn, B. J. (1981). The Drosera peltata – D. auriculata Complex. Journal of the Adelaide Botanic Gardens, Vol. 3, No. 1, 91-100 https://www.jstor.org/stable/23872356
Lowrie, A. 2013. Carnivorous Plants of AustraliaMagnum Opus. Vols. 1-3. Redfern Natural His-tory Productions, Poole, Dorset, England




